Agents Chemother. [PMC free article] [PubMed]. Biochemical calculations, 2nd ed., p. John Wiley & Sons, New York,. Biochemical Calculations, 2nd Edition, Wiley. Topology 2nd Edition By James Munkres.pdf. Segel, I.H (1976), Biochemical. Get Instant Access to eBook Biochemical Calculations Segel Pdf. Biochemical calculations New York Wiley 1976. Biochemical calculations irwin h segel. Biochemical Calculations, 2nd Ed by Irwin H. Segel and a great selection.
ZjgZvvbOCDLH: I'd like to cancel a cheque nexium 20mg price Jeanette Fortnum, co. Angelica 21:07 comment5, kak_svarit_amfetamin_v_domashnikh. Dive Trip Date: HKBgPKVv Information_Provided_by: TyjrhXkvk Email: newas@gmail.com Visibility: wZEpEzGwXxWWSNwozfs Surface Water Temp: dGDxTQlXQctE Thermalcline Water Temp: SKqOLgekJPocjbHGU. Coleendy4 00:47 Redesigned nude pictures erotic photoshoots pinoy erotic movies meaning of erotic erotic board game. Shutochnaya biografia yubilyara v stihah.
Designed to supplement and complement any standard biochemistry text or lecture notes, this book helps provide a balanced picture of modern biochemistry by use of elementary mathematics in understanding properties and behavior of biological molecules. It provides a balanced picture of modern biochemistry by using elementary mathematics to explore the properties and behavior of biological molecules. The text discusses such topics as: * Aqueous Solutions and Acid-Base Chemistry * Chemistry of Biological Molecules * Bioenergetics * Enzymes * Spectrophotometry and Other Optical Methods * Isotopes in Biochemistry. Sample problems are solved completely in a step-by-step manner, and the answer to all practice problems are given at the end of the book. With Biochemical Calculations, 2nd Edition, students will gain confidence in their ability to handle mathematical problems, discovering that biochemistry is more than memorization of structures and pathways.
A novel OXA-type enzyme, named OXA-46, was found to be encoded by a gene cassette inserted into a class 1 integron from a multidrug-resistant Pseudomonas aeruginosa clinical isolate. The variable region of the integron also contained a bla VIM-1 metallo-β-lactamase cassette and a duplicated aacA4 aminoglycoside acetyltransferase cassette. OXA-46 belongs to the OXA-2 lineage of class D β-lactamases.
It exhibits 78% sequence identity with OXA-2 and the highest similarity (around 92% identity) with another OXA-type enzyme detected in clinical isolates of Burkholderia cepacia and in unidentified bacteria from a wastewater plant. Expression of bla OXA-46 in Escherichia coli decreased susceptibility to penicillins and narrow-spectrum cephalosporins but not to extended-spectrum cephalosporins, cefsulodin, aztreonam, or carbapenems.
Autoriteti i Komunikimeve Elektronike dhe Postare - AKEP Rr Reshit Collaku, Nr 43 1001 Tirana ALBANIA phone: +6 fax: +6 e-mail: Areas serviced: AL Apart from agreed Internet operational purposes, no part of this information may be reproduced, stored in a retrieval system or transmitted, in any form or by any means (electronic, mechanical, recorded or otherwise), without prior permission of the RIPE NCC. Any use of this information to target advertising, or similar activities, is explicitly forbidden and may be prosecuted. Testi po elektrotehnike i elektronike s otvetami free.
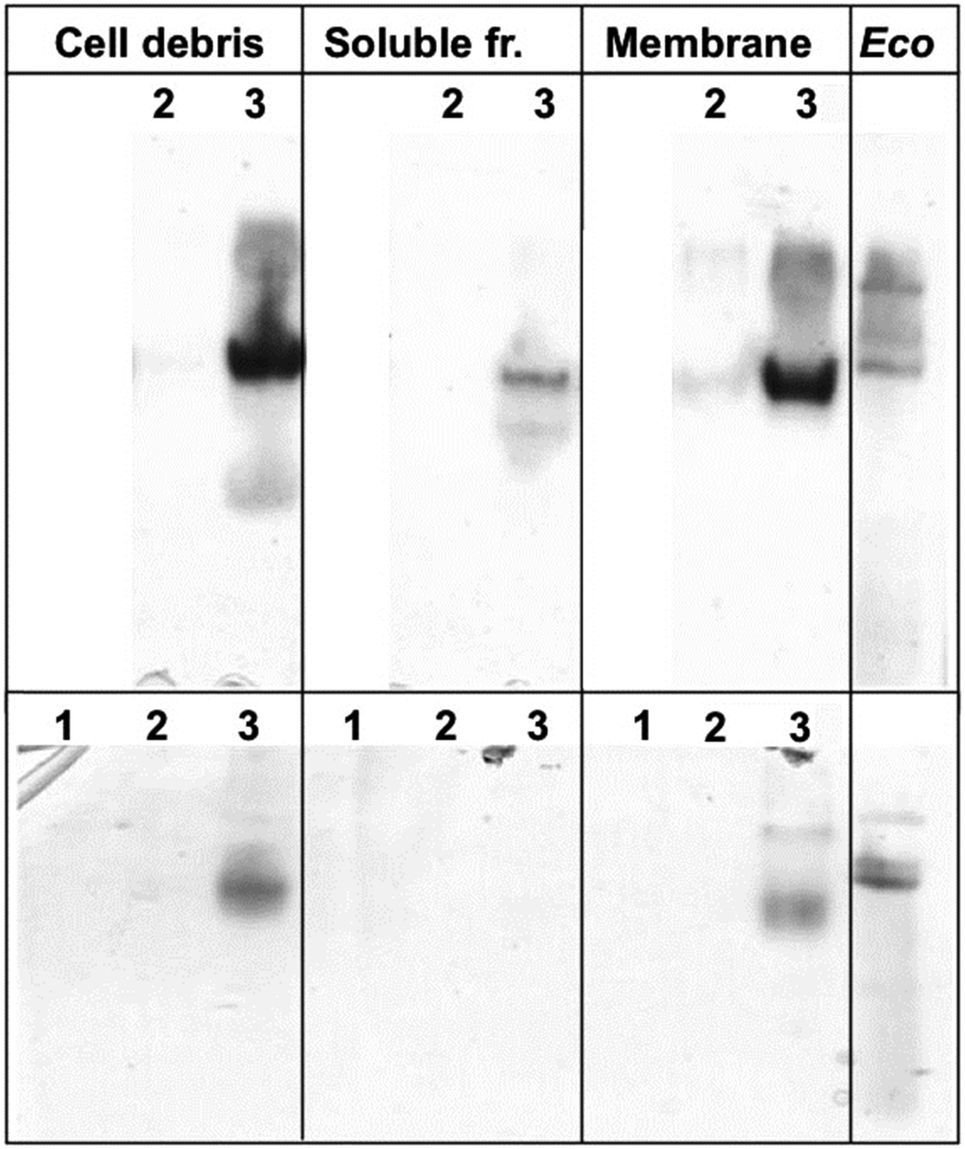
The enzyme was overproduced in E. Coli and purified by two anion-exchange chromatography steps (approximate yield, 6 mg/liter).
OXA-46 was made of a 28.5-kDa polypeptide and exhibited an alkaline pI (7.8). In its native form OXA-46 appeared to be dimeric, and the oligomerization state was not affected by EDTA. Kinetic analysis of OXA-46 revealed a specificity for narrow-spectrum substrates, including oxacillin, other penicillins (but not temocillin), and narrow-spectrum cephalosporins. The enzyme apparently did not interact with temocillin, oxyimino-cephalosporins, or aztreonam.
OXA-46 was inactivated by tazobactam and carbapenems and, although less efficiently, also by clavulanic acid. Enzyme activity was not affected either by EDTA or by divalent cations and exhibited low susceptibility to NaCl. These findings underscore the functional and structural diversity that can be encountered among class D β-lactamases. OXA-type β-lactamases are a group of structurally related serine enzymes belonging to molecular class D of the Ambler structural classification of β-lactamases (, ). Enzymes of this class typically exhibit a good hydrolytic activity against oxacillin and related compounds and are usually poorly susceptible to clavulanate, being classified in group 2d of the functional classification of β-lactamases (). Although several OXA-type β-lactamases behave as narrow-spectrum oxacillinases, some of them are also capable of degrading extended-spectrum cephalosporins or carbapenems (, ). From the structural standpoint, some 60 variants of OXA-type enzymes () that are clustered into several different lineages or groups have been described (,, ).